The Fringes of the Race for a COVID-19 Vaccine
By Saima S. Iqbal, Crimson Staff WriterThe vaccine came together in 10 minutes.
Alone in a private laboratory in Boston, Mass., Preston W. Estep poured five ingredients into a beaker — salt, water, sodium triphosphate, the shrimp-based compound chitosan, and peptides from the novel coronavirus — which together coalesced into gel nanoparticles the size of the SARS-CoV-2 virus.
He poured the mixture into a spray bottle, raised it to his nose, and breathed in the mist.
Estep, then the Director of Gerontology at Harvard’s Personal Genome Project, had long been skeptical of the DIY biology movement— the push to bring biological experimentation outside of institutional settings. But in March, as he followed reports of elderly people dying unattended in nursing homes in Italy and Seattle, he decided not to wait 12 to 18 months more for a commercial vaccine. He believed his community of “leading citizen scientists and edgy academic and company types” could develop a superior product by the end of the year, without ever running a clinical trial or securing billions of dollars in funding: Instead, each member would test the vaccine on themselves.
Alongside three colleagues connected to Harvard Medical School, Estep launched the Rapid Deployment Vaccine Collaborative (RaDVaC) this spring to produce a vaccine based on an “extremely promising” but little-used technology he noticed again and again in research papers.
The team not only designed a novel vaccine — they also released it to the public. In a 59-page report now signed by five Harvard affiliates and 34 anonymous authors, the group lays out the rationale and protocol for its vaccine, disclaiming any guarantees of safety or efficacy.
Their website calls it a “necessary act of compassion.”
For Estep, putting the information online democratized it. “This kind of knowledge, this kind of privilege, is very restricted,” he says. “But if you work a little harder, you can give [people] access to privileges they normally don’t have.”

Two of the group’s co-founders, Alexander Hoekstra and Ranjan Ahuja, worked with Estep on the Personal Genome Project, a Harvard-based study that seeks to sequence and create a public library of the genomes of 100,000 volunteers. HMS Professor George M. Church founded the PGP in 2005, envisioning an undertaking that could accelerate the pace of genetics research and bridge the gap between scientists and citizens.
Upon its founding, the PGP was criticized for posing an undue risk to its participants’ privacy; many doubted that non-scientists could make informed decisions about whether or not to enroll. Today, it’s model is accepted by many — over 10,000 people have participated, and it has inspired offshoots in five separate countries.
Estep, Hoekstra, and Ahuja all drew parallels between the missions of RaDVaC and the PGP, arguing that both endeavors aim to bring science into the hands of regular people. Indeed, the questions the two projects ask are similar: What counts as a valid form of knowledge creation? How should scientists working within the walls of institutions relate to the people beyond it? And where is the line between accessibility and irresponsibility?
Estep hopes people will treat the RaDVaC report less as “the textbook on DIY virology” than as a launching pad for further experiments. He envisions amateur and professional scientists will soon “get under the hood themselves,” tinkering with the design to meet current needs — as well as those presented by future pandemics.
The collaborative initially shared the vaccine with family members, professional connections, and groups of biohackers. In June, as reporters learned that Church — somewhat of a mythic figure in the science world — had also taken it, RaDVaC started receiving heightened attention. Hundreds of people contacted the group expressing interest in the vaccine. The team has since fielded their questions by email and coached some through the protocol in person.
So far, around 50 people have taken at least one dose of the vaccine, with few reporting any symptoms aside from nasal congestion. Most are scientists and physicians, some of whom are at an elevated risk for COVID-19. One department head at a major U.S. hospital had previously considered designing his own vaccine, but found the task daunting. He now plans to modify the RaDVaC vaccine for himself and his wife.
While the group maintains it has a duty to mitigate the suffering caused by the pandemic, many in the scientific community believe its efforts are equal parts futile and irresponsible. Barry R. Bloom, an immunologist at the T.H. Chan School of Public Health, suspects the project will be “absolutely useless” due to its small sample size. More importantly, he fears it will exacerbate the public’s distrust of vaccines. Jacob S. Sherkow, a professor of law at the University of Illinois, says the potential harms are both “easily articulable” and “substantial,” ranging from health complications to false senses of security.
In part due to the outrage, Estep elected to take a leave from the Harvard-affiliated PGP. He remains steadfast in his position, though, and regularly shares a motto with the team: “Don’t be afraid of being controversial. Be afraid of being wrong.” He draws inspiration from Church, his former Ph.D. advisor and long-time mentor, who has routinely ruffled feathers while pushing the boundaries of genetic engineering.
RaDVaC stands out even among other citizen science projects for its ambition (and risk and prestige). At its core, however, it is driven by the same belief that governs efforts to produce cheap insulin and imagine new models of research like the Personal Genome Project’s: that our scientific institutions are not only slow-moving but dangerously insular.
‘Easier Than Your Average Brownie Recipe’
On March 9, Estep sent a mass email to a list of associates he believed might be interested in working on a DIY vaccine. US government estimates that a therapy would take over a year to develop seemed painfully slow. “There is already sufficient information [online] to guide the development of a safe and likely effective vaccine,” he wrote.
Some responded, Estep remembers, along the lines of, ‘This sounds crazy, I don’t think I can be involved with it.”
But a few others adopted a different tack: “This sounds crazy; let’s definitely do it.”
Hoekstra, a former project manager at the PGP, read the message while attending what was to be his last in-person scientific conference. At first, he kept picturing the challenges they’d face producing a vaccine. But the more he thought about it, the more he was drawn to the idea. By mid-March, the pandemic had already spun out of control. Hoekstra felt that even if the project failed, it was “irresponsible not to act.”
Hoekstra decided to join Estep and two others— Ahuja, Director of Community at the PGP and Don Z. Wang, an HMS-trained immunologist— in co-founding RaDVaC. The four soon began their project in a rented laboratory unaffiliated with Harvard, working with commercially available reagents.

Within three weeks, the team had settled on an initial version of the vaccine.
Estep administered it to himself on March 28. He was skeptical, but says he had no reason not to take it — he didn’t believe it was dangerous. And as soon as he inhaled the solution, he felt he had taken a big first step towards “something important.” “I had this incredible psychological lift,” he recounted. “Every time I take it, I have that feeling.”
When he developed a stuffy nose later that afternoon, he took it as a promising sign.
The other three followed suit in April and June, taking one dose of vaccine every two weeks. When I spoke to Hoekstra, he said he was “already looking forward” to his next one. “It’s got some good CD4 T-cell epitopes in there,” he added, smiling.
In early July, the group published a white paper on its website detailing the specifications of its vaccine so others could make it themselves. Estep, Ahuja, Hoekstra, and Wang signed on as co-authors; a dozen other initial participants elected to remain anonymous.
According to Estep, it typically takes lay people around 25 minutes to make the vaccine. The recipe requires little lab equipment, and one can procure both the ingredients and materials required to make it from biotech sites and Amazon for less than $1400 in total.
Reading the protocol, I was surprised to see that it was simpler than some of the procedures I’d worked on in freshman year biology courses.
Soon after the publication of the white paper, Church enlisted as a guinea pig. For many observers, his choice brought increased attention, and scrutiny, to the project’s methods.
Onlookers have criticized RaDVaC for forgoing the typical model of a clinical trial, which usually consists of four stages that take place over the span of several years, with different stages testing for safety, efficacy, and rare side effects.
Estep and Ahuja claim this system is ill-suited to adapt to emerging pandemics, and that there are several reasons their model is superior to that of a clinical trial.
For one, they argue clinical trials don’t allow for vaccines to adapt to new research findings. Many of the vaccine designs currently in clinical trials were locked in when we knew less about COVID-19. Most of those vaccine candidates rely on similar strategies — delivery via injection and targeting of the spike protein, the receptor the novel coronavirus uses to enter our cells — risking mass failure. RaDVaC’s vaccines, by contrast, are delivered via nasal spray and include other peptides that might elicit an additional form of immunity; Estep and his team also adapt the RaDVaC vaccine as new information about the coronavirus emerges.
Church’s justification of RaDVaC is a bit more cautious. He says he believes the pandemic has opened the opportunity to try to answer the question of how to rapidly deploy a vaccine in times of crisis. Though he emphasizes the importance of clinical trials, he nonetheless believes the RaDVaC vaccine is safe in small numbers and will be taken largely by people who understand its risks.
He anticipates the vaccine will soon move to a study, where it will be regulated by conventional standards. He sees this informal experiment as a means to bring the team’s design into the running, since clinical trials often require billions of dollars.
Church isn’t taking the vaccine out of fear that he will contract the virus — he hasn’t left his home in five months. He is a true believer in RaDVaC’s mission. He thinks the project may demystify vaccines for those who are wary of them; he also believes the model of open access will get people excited about and engaged with science and realize “facts don’t just come from the sky.”
So far, Estep claims, the RaDVaC model has been safe. Church says that “it’s riskier to go to a supermarket than to take a few peptides by the nose.” Of the about 50 people who have taken the vaccine, Estep says, the most common symptom participants have exhibited is nasal congestion — which, again, the RaDVaC team takes as a positive sign, Estep explains, apologizing to me for sniffling as we speak.
It remains unclear, though, whether the RaDVaC team can generate meaningful conclusions from self-experimentation. Bloom argues that their studies will not be able to give them any conclusive results, since rare side effects only emerge in larger sample sizes.
But regardless of whether or not their data is meaningful, many critique how they obtain it.
The white paper detailing the instructions for making the RaDVaC vaccine is available to anyone on the internet. Estep claims that, because they outline the potential risks in the same report, anyone who takes the vaccine is participating in a model of “informed consent” — they have the information they need to make an informed decision about whether to participate.
But others, like Sherkow, question whether an average person, desperate for a cure and trusting of the Harvard name, can be expected to give informed consent. And even if they can, what happens if they make a mistake in assembling the vaccine? What happens if, as a result of taking the vaccine, they change their behavior in regards to social distancing? What if they decide not to sign up for clinical trials or take a commercial vaccine later?
Responses to questions of what exactly constitutes “informed consent” in this context vary. Hoekstra believes the specialized language in the white paper will limit the population of people who engage with the vaccine instructions to those who are “scientifically literate.”
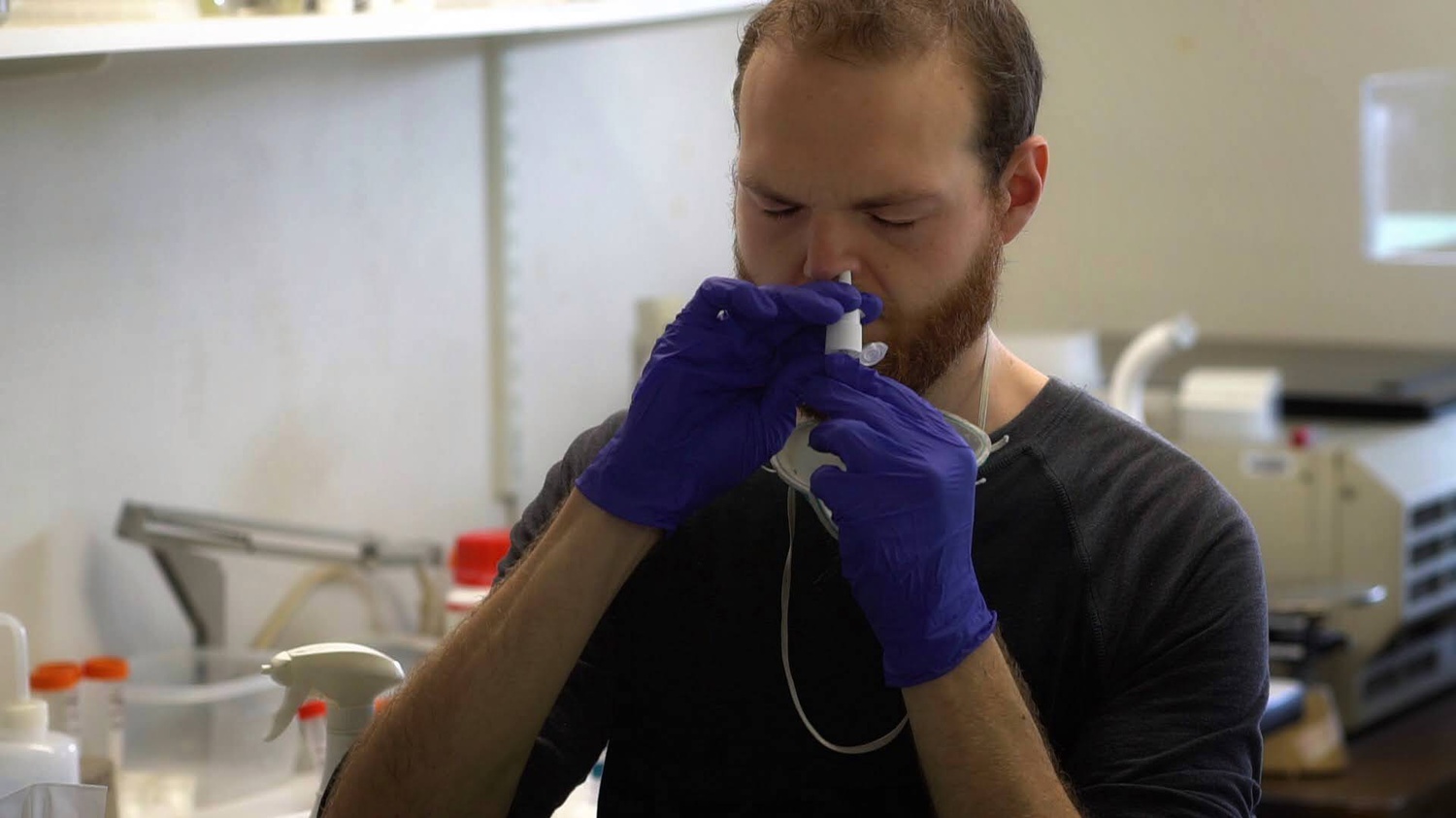
Church puts it differently: “We discussed the possibility of publishing a little piece of it just as if it were a cookbook,” he says. “Like, ‘This is how you make brownies. And this is how you make RaDVaC. And oh, by the way, the RaDVaC recipe is a little bit easier.’ No, that's not the way we did it. We actually want people to, if they want it badly enough, they will read about it.”
Estep seems more optimistic about the idea of non-scientists benefiting from RaDVaC — he claims that the jargon in the report is not an insurmountable barrier, but simply a hurdle for those interested. “We all start off as non-scientists,” he says.
The group also rejects the notion they are recruiting participants or providing advice; they believe they are simply putting information online and allowing people to respond as they will.
The issues of informed consent, of bridging the gap between the general public and the ivory tower of scientific research, have their roots in the Personal Genome Project. Three of the four RaDVaC co-founders were members of the PGP, and several of them drew analogies between their work at the PGP and their work at RaDVaC.
Hoekstra called the PGP “an act of re-empowering people to contribute their data to something much larger than a single project,” and claimed that the philosophies of the PGP “translated very easily and really helped empower or shape what RaDVaC became.”
Estep said that the motivations behind the PGP — and his participation in science in general — are rooted in increasing citizens’ “engagement in real-world science.” He says, “This is something that George Church and I have always sort of been aligned on, and that he’s really pioneered: creating infrastructures for people to contribute scientifically outside of the normal channels.” In Estep’s eyes, RaDVaC is another one of those infrastructures.
Estep also says the PGP has helped him to think about how to responsibly operate RaDVaC. “PGP has given us a unique perspective and the deep understanding of how to conduct citizen science,” he says. “We know the complexities of the kind of open and informed consent that we’ve employed at RaDVaC.”
So who is “science” for, really?
‘The Most Out-There Stuff We’ve Got’
In 1993, time was running out for Estep to settle on a laboratory for his Ph.D. thesis. He had found his past two rotations unfulfilling, and hoped, with the third, to finally find a place where he could combine his interests in biology and computer science — cutting-edge stuff for the time.
His advisor said one man might fit the bill: a young assistant professor named George Church. “If he’s not weird enough for you, then there’s nothing for you at Harvard,” Estep remembers her saying. “This is the most out-there stuff that we’ve got.”
The first time he heard Church speak, Estep was “in heaven.”
“It was the most mind-blowing stuff I’d ever heard,” he recounts. Within five minutes of meeting Church, he knew he wanted to work with him. He thought, “my life is changing; my life has just changed.” Church has had a “profound influence” on him as a scientist and as a person. “I don’t remember the old me anymore,” he says.
Church is something of a celebrity in the world of genetics. The details of his biography paint a picture of a man who has never followed the rules— after graduating from Duke in two years, he flunked out of a graduate program for spending too much time in the lab and forgoing his coursework. But he was later accepted to Harvard’s graduate school, where, in 1984, he devised the first-ever direct DNA sequencing technology.
In 2005, when Church had been a professor at Harvard for 19 years, he had the idea to sequence the genomes of 100,000 strangers. At the time, the ethical standard for genomic research was to recruit a cohort of people to study one condition, get them to sign a consent form, promise not to use their data for anything else, and then never contact them again. Because researchers needed to protect the privacy of their participants, they couldn’t ask for too much information and they couldn’t share data sets. If they learned anything that could be of use to the participant (such as a genetic risk), they weren’t allowed to communicate it.
Church felt this was absurdly inefficient and pointless, especially because many scientists knew they could not truly protect the privacy of their study participants — the information they gave was enough to be identifying — and yet relied on boilerplate consent forms anyway. Having participated in many psychological studies himself as an undergraduate, he felt transparency would breed trust.
He imagined researchers could upload participants’ genomes and medical records for wide public use. Instead of one research team keeping the data to themselves, they could share it in the hope that others would use the data to study all sorts of conditions. Breaking down the barrier between researcher and participant could be a win-win situation: In exchange for sending their biological samples and health records to various scientists, participants could then learn of any genetic risks they might have.
Church received a $10 million grant from the National Institute of Health to move his experiment forward — but when Harvard Medical School’s Institutional Review Board looked over his project, they paused it out of concern. The board feared that few participants would enroll in the study, which would open up the possibility of genetic discrimination by empoyers or insurance companies. Church says this worry has proven to be unfounded; such discrimination is not only now illegal but quite rare.
Church believes people were so hesitant to adopt his model for two main reasons: first, because Harvard feared the liability that might come from disclosing (incorrectly or correctly) someone’s risk factors, and second, because there was inertia among researchers who he claims had their own “fiefdoms” — they had worked hard to acquire their own data sets and didn’t want to share them.
Alongside an ethicist and a lawyer, Church created a new, open model of consent for this project, which became the Personal Genome Project — a model that is widely-accepted today. Participants have to receive a perfect score on a thorough assessment that tests whether they understand the potential risks of putting such information online.
Estep says it was only later that he fully realized the benefits of the open-consent model — it allows people to engage with the research, he claims, which not only makes them “more effective research subjects,” but also “more educated” citizens.
In describing the difference between the model of open consent and more traditional research models, Estep draws a comparison with RaDVaC, which similarly seeks to “empower” people with information, rather than “the very conservative and patriarchal biomedical practice people usually engage in where they’re like, ‘Okay, I’m going to stick this needle here and inject you with something. Trust me, It’s good for you.’”
It took a year for HMS’s Institutional Review Board to approve the project. It rolled out in phases, with the first volunteers being Church and other medical experts the Board believed could make informed decisions. In an interview conducted at the time, Church said the project “could be something very big once people tune in; not many people know about it so far.”
Today, more than 10,000 volunteers have joined the project, and it has inspired offshoots in five countries. Church and his family even did a road trip across Canada to meet participants who wrote to him about joining the study.
In 2012, Hoekstra was an undergraduate student at Michigan State University who had recently become so disillusioned with conventional scientific research — what he saw as a “toxic relationship” between scientists, their institutions, journals, and funders — that he considered forgoing plans to attend graduate school.
But then he learned of the PGP — then described as a zany project going on in Cambridge — in a footnote in a lecture on genomics. He was so captivated by it that he decided on a whim to come up to Boston and attend a PGP conference.
At the conference, he met Church and expressed his concerns about the scientific establishment.
“George pulled on his beard for a moment and said, ‘If that’s how you feel, you shouldn't continue, you should do something different. And if you'd like, you should come out and work on the Personal Genome Project,’” Hoekstra recalls. “That changed my life, changed my whole trajectory.”
It was so life-changing, Hoekstra says, because he finally found people who were willing to challenge the status quo. He believed he was among people who embraced “the idea of doing things differently, doing things that seemed radical, and to many people seem[ed] incredibly risky,” but that “had the power to change the way science is done and change the way we understand ourselves and the world.”
Ahuja describes meeting Estep and Hoekstra as a “blessing.” He felt an “instant connection” to them, which he says is “very rare” given his eccentric outlook. Working with them, he feels he can implement “ideas that have a chain reaction,” “ideas that change not just one thing but change the conditions going forward.”
Estep says that the spirit of the PGP — its commitment to engaging citizens in science and disrupting traditional models of science — persists in RaDVaC. One reason he decided to post the vaccine design online was that doing otherwise would be “completely contradictory to [his], and George Church’s, commitment to intellectual sharing and open science.”
Beyond the ‘Walled Garden’
Sherkow, the bioethicist, finds this comparison between the PGP and RaDVaC incomprehensible. He calls the PGP the “mirror image,” the opposite, of RaDVaC. Whereas the PGP requires a high standard of consent for a low risk project that is low risk, Sherkow says, RaDVaC sets a low standard for a high risk project. If people can mess up baking bread, he argues, they can certainly mess up manufacturing this vaccine.
And where the PGP, to some, met a real need, Bloom, the HSPH immunologist, believes scientists are already deploying a vaccine as fast as they safely can.
Whereas Church finds the analogy between the PGP and RaDVaC somewhat apt, others at the PGP have distanced themselves from the vaccine and declined to comment for this article. Jeantine E. Lunshof, the ethicist that helped Church conceive of the open consent model has called RaDVaC an “ego trip.”
And while Church’s lab has passed scrupulous institutional review, RaDVaC does not report to any regulatory body, and it does not yet require self-experimenters to report symptoms. All such steps in the protocol outlined in the white paper are labeled as “optional.” While the team does plan on creating a platform for people to discuss their experiences with variations of the vaccine, it is unclear what this might look like and when it will be open to the public.
Bloom also suspects few people will self-administer the RaDVaC vaccine, given that several commercial vaccines are likely to hit the markets by early next year.
The project exists in a murky legal space. The team could be subject to FDA crackdown if the FDA determines they are distributing the vaccine or its ingredients across state lines, though Sherkow says he believes this is unlikely, since the FDA so far has not taken any action against DIY COVID vaccines. Sherkow adds that because DIY biology has never been so common or so sophisticated, there aren’t many laws regulating it.
Scientific institutions have long struggled to balance profit and progress. Even today, Jones points out, people struggle to access the medications they need because pharmaceutical companies abide by commercial incentives, monopolizing certain drugs and not working on rare diseases. Some people who are drawn to DIY bio are simply desperate for another way out. By extending the same logic to COVID-19 vaccines, this team of Harvard scientists seems to suggest it’s time to work outside the constraints of the pharmaceutical and research landscape, and in the process are raising the question of what risk and responsibility mean in these circumstances.
Whether the RaDVaC team succeeds in their effort or not, they have at the very least attracted the interest of two startup biotech companies. While they have yet to sign any contracts, they are in advanced talks about moving a vaccine based on their original design, backed by a small amount of evidence, into a clinical trial.
Still, Estep and Ahuja stress that the initial goal of RaDVaC was not commercialization. Instead, Estep says, the project was borne out of the group’s commitment to take science out of its “walled garden,” to make its findings accessible to the public, to engage as many people as possible in the process of scientific discovery — despite the torrent of criticism.
Ahuja remembers that as he first prepared the vaccine, many thoughts rushed through his head. He thought about how his actions reflected a commitment to “participate in a certain path of inquiry” and to “push the bounds of scientific understanding.” He imagined what their effort represented for “science itself as an enterprise.” He felt a sharp sense of “duty” and “privilege” to “[help] move the practice of science forward.”
Then, he took a deep breath in.
Correction: October 12, 2020
A previous version of this article incorrectly stated that Preston W. Estep worked out of a laboratory in Cambridge, Mass. In fact, the laboratory was in Boston.
Correction: October 12, 2020
A previous version of this article incorrectly stated that RaDVaC coached people through their vaccine protocol by email and phone. In fact, they did so by email and in person.
Correction: October 12, 2020
A previous version of this article incorrectly stated that the ingredients and materials required to make RaDVaC’s vaccine cost roughly $200. In fact, it cost roughly $1400.
Correction: October 12, 2020
A previous version of this article incorrectly stated that nasal congestion was the worst symptom vaccine-takers experienced. In fact, it was the most common symptom.
Correction: October 12, 2020
A previous version of this article incorrectly stated that Alexander Hoekstra was a graduate student at Michigan State University in 2005. In fact, he was an undergraduate in 2012.
Correction: October 12, 2020
A previous version of this article incorrectly stated that RaDVaC received interest in their project from pharmaceutical companies. In fact, they received interest from biotech companies.
— Staff writer Saima S. Iqbal can be reached at saima.iqbal@thecrimson.com.